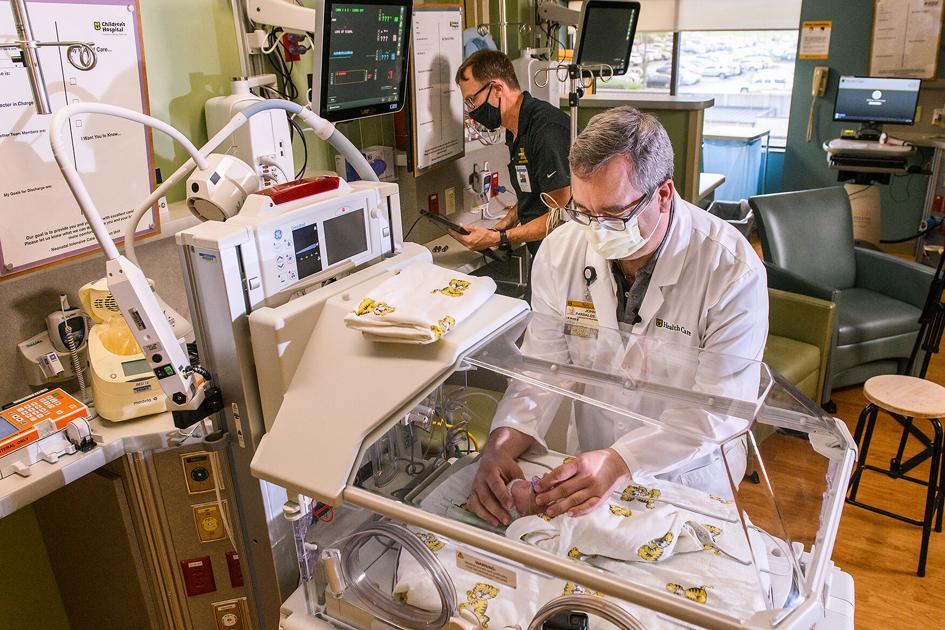
An MU researcher has created a device that could help premature babies get the oxygen they need in the critical first few weeks of life.
After birth, many premature newborns are sent to the Neonatal Intensive Care Unit. There, care is provided around the clock with equipment to help monitor the infants. Such care includes regulating oxygen levels, a task that involves numerous adjustments to dial in just how much oxygen the infant needs.
For a premature infant, saturated oxygen levels must remain between 88% and 95%. If oxygen levels are too low, the baby could have trouble properly developing. If the oxygen levels are too high, the newborn will develop a disease called retinopathy of prematurity, which causes blindness.
Currently, a nurse is required to help regulate how much oxygen the newborn is receiving, which not only puts a strain on nursing staff, but can also deter the positive outcomes of newborns in the NICU.
Roger Fales, associate dean of student services for MU’s College of Engineering, and his team have developed a device that reads the oxygen level of the infant and makes adjustments as necessary.
The clinical trial will use the device on 60 newborns, half from Women’s and Children’s Hospital and the other half from Studer Family Children’s Hospital at Ascension Sacred Heart in Pensacola, Florida.
Around 2008, Fales started modeling data of infants in the NICU, gathering information on the parameters that a device such as his would need to meet. Then, he and his team started working on the device, something that would be able to fit the parameters needed.
Funding for the project came from MU’s Coulter Translational Partnership Program and the National Institutes of Health.
Development has led to the device as it is now, a microcontroller adjusting oxygen and using an algorithm to learn and adjust to meet the immediate needs of the newborn. The device is a motorized dial attached to the machinery that provides oxygen to the baby in a hospital setting.
To compare oxygen regulation to the traditional method, the device will be used for six hours on the patient, then a nurse will regulate the oxygen by hand for six hours. This cycle will continue for six days. For the trial to be deemed successful, the device will need to regulate oxygen levels similar to, if not better than, the current method.
Working on the device has been a longtime collaboration between the College of Engineering and the MU School of Medicine. Fales is currently working with John Pardalos, an associate professor in the Department of Child Health in the School of Medicine. Pardalos emphasized the importance such a collaboration can have on students helping with research and development.
“For an engineering student that is working on medical devices to be able to actually see them in use on the patient, it will help them in the future and in their careers,” Pardalos said.
Fales said this collaboration not only has an effect on the students, but is what led to the creation of the device.
“Without having the ability to collaborate with good people at MU Health Care and the MU School of Medicine, this sort of collaboration would not be possible,” Fales said in a news release. “It really takes both engineers and medical doctors to be able to put this kind of project together.”
"device" - Google News
September 22, 2021 at 04:30AM
https://ift.tt/2XE5dVC
Device regulating oxygen in premature infants goes to clinical trials - Columbia Missourian
"device" - Google News
https://ift.tt/2KSbrrl
https://ift.tt/2YsSbsy
Bagikan Berita Ini














0 Response to "Device regulating oxygen in premature infants goes to clinical trials - Columbia Missourian"
Post a Comment