DeepBio said its DeepDx-Prostate Pro, an artificial intelligence (AI)-based pathology diagnosis assistance software, has received a Class 3 in-vitro diagnostic medical device from the Ministry of Food and Drug Safety.
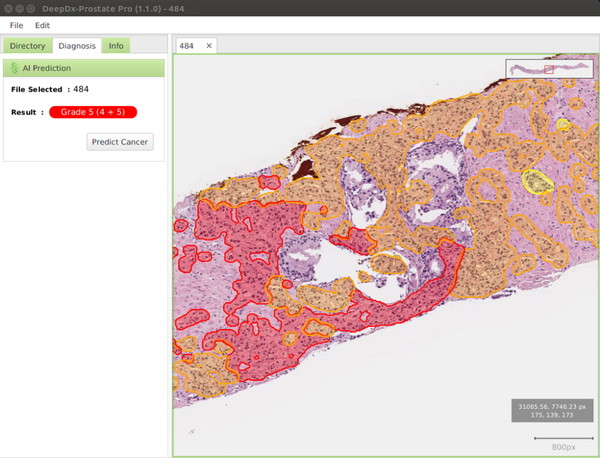
According to the company, the approval marks the first AI-based medical device that assists in classifying prostate cancer severity in the world. The company had also received approval for AI-based prostate cancer diagnosis assistance software for the first time in Korea in April last year.
The company could receive approval for the device after demonstrating excellent performance in an evaluation study. DeepDx-Prostate Pro showed a 98.7-percent grade group classification agreement and 96.9-percent no grade group classification agreement.
As a result of comparing the analysis of this software with the analysis of three pathologists in the same study, DeepDx-Prostate Pro recorded a Kappa statistic of 0.713 and a weighted Kappa statistic of 0.922 indicating high agreement with the pathologist.
The device also drastically shortened the time needed for the analysis. While it took 550 minutes for a pathologist to analyze the slides of an entire sample through an existing method, the device greatly reduced the time needed to 364 minutes.
"It is very meaningful that DeepBio introduced the world's first AI technology that aids in pathological tissue diagnosis," Deep Bio CEO Kim Sun-woo said. "In the diagnosis of prostate cancer, the severity diagnosis according to the Gleason classification method is very important for patient prognosis and treatment, and it is one of the areas requiring AI diagnosis support due to frequent diagnosis discrepancies between pathologists."
Following the launch of an auxiliary medical device for diagnosing the presence or absence of prostate cancer with deep learning technology last year, the company expects that the new approval will enable faster and more accurate diagnosis of prostate cancer, Kim added.
DeepDx-Prostate Pro automatically classifies the histological severity of prostate cancer by analyzing whole slide images of prostate needle biopsy tissue stained with Hematoxylin & Eosin with AI.
The system provides the analysis results in five grades and a Gleason score based on the Gleason grading system, which is the most used method for classifying the degree of differentiation of prostate cancer tissues.
"device" - Google News
November 15, 2021 at 09:59AM
https://ift.tt/3on2utm
Regulator OKs DeepBio's AI-based prostate cancer pathology diagnosis SW - Korea Biomedical Review
"device" - Google News
https://ift.tt/2KSbrrl
https://ift.tt/2YsSbsy
Bagikan Berita Ini














0 Response to "Regulator OKs DeepBio's AI-based prostate cancer pathology diagnosis SW - Korea Biomedical Review"
Post a Comment