LEXINGTON, Mass., March 4, 2021 /PRNewswire/ -- Cognition Corporation, a leader in SaaS solutions for guided compliance in medical device and pharmaceutical product development, has released a new suite of industry-leading risk management functionality in its Compass® solution. Compass' risk management feature includes guided risk analysis supported by regulatory standards, sequence of events management, and native integration with requirements and test management to ensure quality is integral throughout all phases of product development. Cognition's risk-first approach places emphasis on comprehensive risk analysis and its integration in the design control process so that all risks, whether they are the result of a failure or not, are identified and considered.

"Risk analysis done properly is complex, as is managing risk within the design and development process; however, if not done properly, the result could be harm to the patient. That is why we've purposefully built software that makes it significantly easier for development teams to conduct a thorough, completely traceable, risk analysis that is tightly integrated with requirement and test management tools," said Ben Higgitt, Compass Product Line Manager.
Compass brings best-in-class functionality for better risk management and safer products, including:
Guided Risk Analysis with Regulatory Standards and the Risk Management Plan
Compass guides users through the risk analysis process, and enforces policies based on regulatory standards like ISO 14971 and the project's risk management plan. By developing products alongside standards and in accordance with an overall risk management plan, compliance can be rapidly achieved. By focusing on best practices, documents will be audit and submission ready.
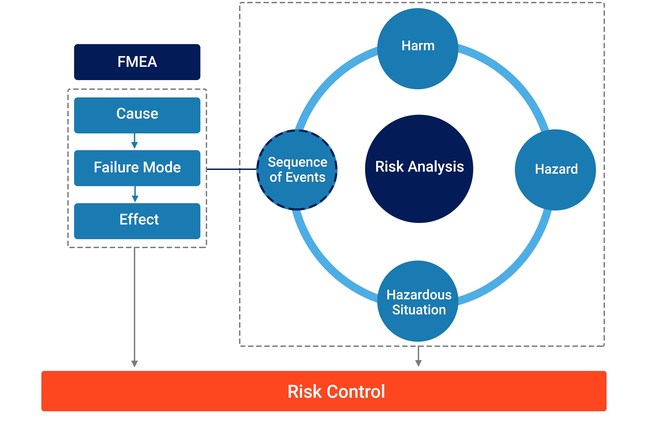
Sequence of Events Management
Compass not only manages individual risk data points, like hazards, hazardous situations, and harms, but it also manages the sequence of events that could ultimately lead to a patient being harmed. Connecting individual risk data through a 'Sequence of Events' enables all risks, whether they are caused by a failure or not, to be identified and mitigated.
Native Integration with Requirement and Test Management
In Compass, risk management is tightly integrated with requirements and test management to assess the impact of every change across every function. This enables mitigations and controls to be implemented with a clear, traceable, audit trail across all functions.
For more information on Compass' risk management click here.
About Cognition Corporation
Cognition develops, sells, and supports risk-first product development and compliance solutions for the medical device and pharmaceutical industries and is trusted by the world's leading life sciences companies. Its Software-as-a-Service platform enables customers to structure their data and automate processes with built-in quality to save time and money and bring products to market faster. For more information, visit www.cognition.us.
Press Contact:
Matt Burke
+1 603.315.0618
[email protected]
SOURCE Cognition Corporation

Related Links
"device" - Google News
March 04, 2021 at 08:44PM
https://ift.tt/3bh9qD8
Compass Delivers Industry-Leading Risk Management for Medical Device Development - PRNewswire
"device" - Google News
https://ift.tt/2KSbrrl
https://ift.tt/2YsSbsy
Bagikan Berita Ini














0 Response to "Compass Delivers Industry-Leading Risk Management for Medical Device Development - PRNewswire"
Post a Comment